📦FREE SHIPPING ON ALL ORDERS🍄 Dismiss
Skip to content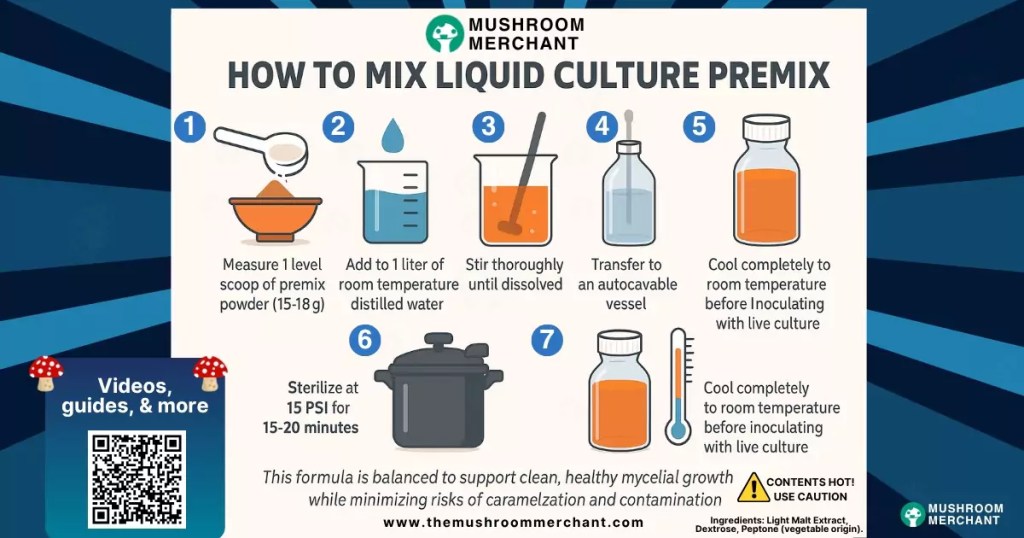
To prepare 1 liter of liquid culture (LC) media:
This formula is balanced to support clean, healthy mycelial growth while minimizing risks of caramelization and contamination. This product has been formulated by professional mycologists to have consistent results for a wide variety of species. Scale volume up or down as needed. Liquid culture premix instructions are intended as a general guideline.
Always use caution when working with hot materials and use appropriate personal protective equipment (PPE) at all times. The customer is responsible for safe use of all products. Company, manufacturers, owners, or sellers take no responsibility for misuse of this product.
Inoculating Liquid Culture with Liquid Culture
Liquid culture can be expanded by transferring mycelium from one LC to another. This is commonly called “LC to LC.” While this method can save time and rapidly expand your culture supply, it carries a higher risk of spreading contamination if sterility isn’t maintained. Always perform LC-to-LC transfers in front of a flow hood or inside a still air box (SAB) for best results. Technically, it can be done without such equipment, but it’s strongly discouraged since any hidden contamination will be amplified in the new culture. Use sterile syringes and make only small transfers (1–2 mL) to reduce the risk of introducing contaminants.
Inoculating Liquid Culture with Agar
The most reliable way to start a new liquid culture is from a clean agar plate. Once you’ve isolated healthy mycelium on agar (free of contaminants), you can transfer it into sterile liquid culture to expand it. Using a flow hood is strongly recommended for this process, though a still air box (SAB) can also work if you don’t have one. Cut a small wedge of clean mycelium from the agar plate using a sterile scalpel, and drop it into the LC jar or vial. The mycelium will grow outward into the nutrient solution, colonizing it over time. This method ensures that your LC begins from a verified, clean source and greatly reduces the risk of contamination compared to spore syringes or LC-to-LC transfers
Inoculating Liquid Culture with a Spore Syringe
Although it is possible to inoculate liquid culture directly with spores, this method is not recommended. Spore syringes often contain bacterial or competing organisms that are invisible to the eye but will flourish in liquid culture. Instead, spores should first be germinated on agar, where healthy mycelium can be isolated away from contaminants. Only after you have a clean culture should you consider transferring it into liquid culture. If you do attempt spore-to-LC inoculation, always use a sterile workspace (flow hood or SAB) and treat the resulting culture with caution until verified on agar.
Inoculating Agar with Liquid Culture
Transferring liquid culture to agar is one of the best ways to check its purity and ensure you’re working with clean mycelium. Place agar plates inside a flow hood or SAB, flame-sterilize your syringe needle, and apply 1–2 drops of liquid culture to the surface of the agar. The mycelium will begin to grow outward from the inoculation points, allowing you to assess its health and identify any contaminants. This step is highly recommended before scaling up to grain, as it acts as a “checkpoint” for culture quality.
Inoculating Grain with Liquid Culture
Once you’ve confirmed that your liquid culture is healthy and clean, it can be injected directly into sterilized grain bags or grain jars. For each standard 3–5 lb grain bag, use 3–5 mL of liquid culture. Larger bags can take up to 10 mL. Always flame-sterilize the syringe needle before injection. If you have a flow hood or SAB, use it — but if not, our grain bags come with self-healing injection ports that allow for a safer inoculation without open-air exposure. Simply inject the culture through the port, withdraw the needle, and mix the bag gently to distribute the liquid evenly. This ensures strong, even colonization across the grain.
Liquid Culture Storage
Once your liquid culture is fully colonized and ready for use, it should always be stored in a refrigerator between 2–8 °C (35–46 °F). This cool, stable environment slows the mycelium’s metabolism, extending its shelf life while keeping it viable for future inoculations. Cultures should be kept in sterile, airtight, autoclavable containers and placed away from light to prevent degradation or contamination. Under these conditions, liquid culture can remain healthy and usable for 6–12 months, making proper storage just as important as the preparation process itself.
How to Make Liquid Culture FAQ
Use 15 to 18 grams of liquid culture premix per 1 liter of distilled water.
No. Use distilled water to avoid introducing minerals or bacteria that may compromise the culture.
The vessel may rupture or pressure may build dangerously. Always keep lids loose during pressure cooking/sterilizing.
Not required, but it helps ensure a more uniform solution. Manual stirring works if thorough.
It is crucial to cool media to room temperature. Hot media can kill spores or mycelium. Always wait until the jar is fully cool to the touch before adding live culture.

Learn More About Mycology
Get updates and learn about growing mushrooms